High blood pressure, or hypertension, is a global health challenge affecting over a billion people. While many can manage their condition with lifestyle changes and existing medications, a significant portion of the population suffers from “resistant hypertension”—a form of high blood pressure that remains dangerously elevated despite taking multiple medications. Baxdrostat, an investigational drug developed by AstraZeneca, is emerging as a potential game-changer in this field.
What Is Baxdrostat Treatment?
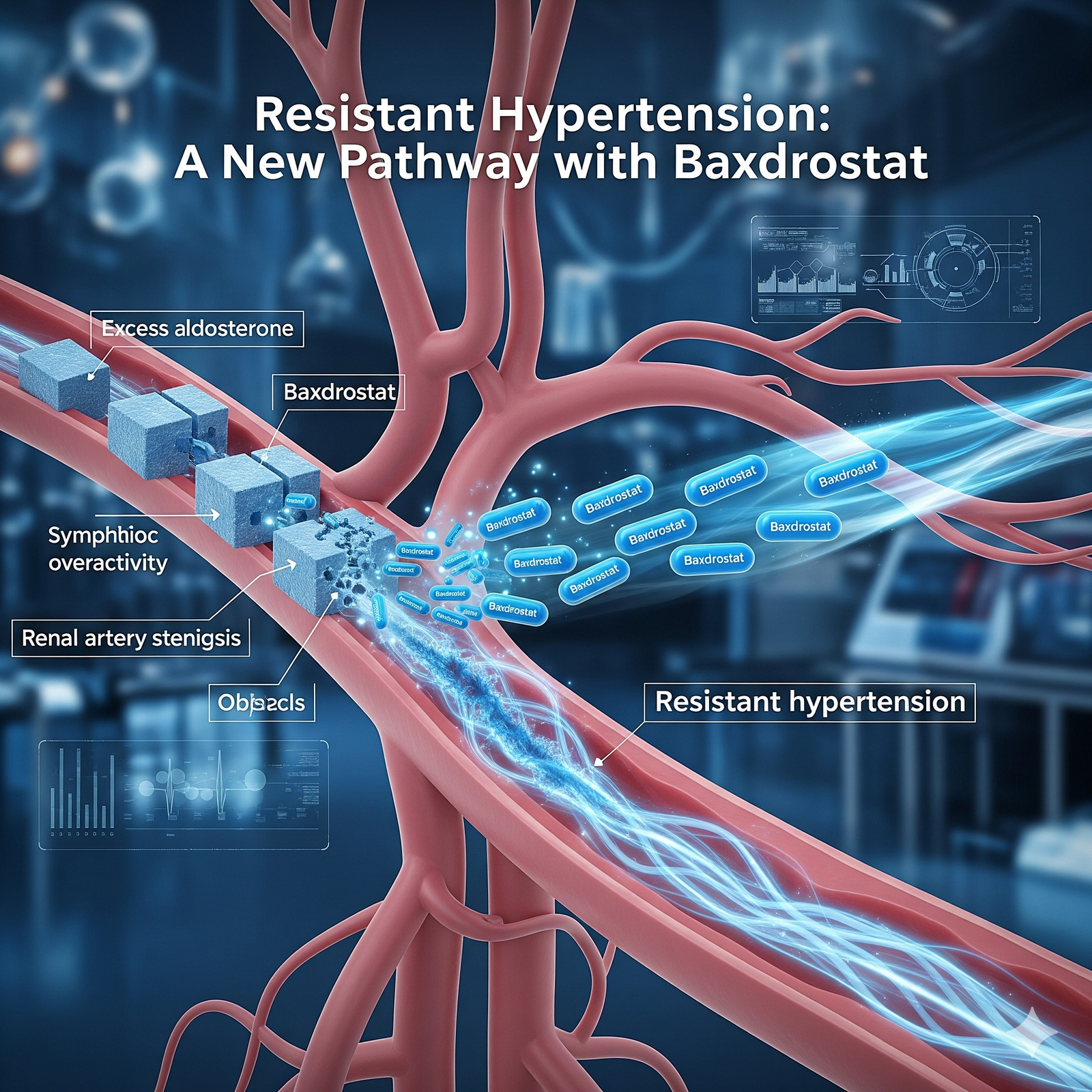
Baxdrostat is a novel drug designed to target specific biological pathways involved in disease processes, offering a promising therapeutic option for patients. As research advances, healthcare providers and patients alike are eager to understand its full potential.
The Mechanism of Action
The mechanism of action of Baxdrostat medication is centered around its ability to selectively inhibit enzymes or receptors that play a critical role in disease progression. By targeting these pathways, the experimental therapy helps regulate abnormal biological activity, potentially reducing symptoms and improving patient outcomes. This targeted approach aims to provide effective treatment with minimized side effects compared to broader-acting therapies.
Clinical Trials and Promising Results
Clinical trials for Baxdrostat medication have been instrumental in evaluating its safety and effectiveness. These studies involve diverse patient populations and are designed to assess how well the drug performs in real-world conditions. Initial trial results have shown promise, indicating that Baxdrostat can effectively address the target condition with manageable side effects. Continued research and larger trials are in progress to confirm these findings and to explore additional uses for the drug.
Side Effects and Safety Profile
Like any medication, the novel hypertension drug drug may cause side effects:
- Commonly reported side effects during clinical studies are mild to moderate.
- Most side effects can be managed with guidance from a healthcare provider.
- Serious side effects are rare but are carefully monitored in ongoing trials.
- Understanding potential side effects is important for patients considering AstraZeneca’s blood pressure drug drug treatment.
Availability and Regulatory Status
The positive results from the BaxHTN trial have paved the way for regulatory filings. AstraZeneca has stated its intention to advance regulatory submissions with health authorities in the coming months. While specific approval and launch dates are not yet confirmed, the successful Phase III data suggests a potential for regulatory approvals in the near future. Information regarding its availability in Australia or other specific regions is not yet public, but global regulatory submissions are expected.
Conclusion
The emerging hypertension treatment represents a hopeful advancement in medical treatment, with a clear mechanism of action, promising clinical trial results, and growing interest in markets like Australia. While pricing and availability details are still being finalized, patients and healthcare providers eagerly await its arrival. Continued research and regulatory progress will determine how soon the investigational drug can become a mainstream option for those in need.